CONTRIBUTING TO RESEARCH
The Path To Advancing Medicine and Changing Lives
JOIN A FDA APPROVED CLINICAL STUDY!
SIGN UP TODAY- Space is limited!
Are you someone who likes to contribute to society? If so, you may qualify to participate in one of our FDA-approved clinical studies. All qualified participants will receive compensation for time and travel, study-related medication and complementary doctor visits! Registration is easy…convenient…and it's happening right now.
HERE'S HOW TO GET STARTED
As a study participant, you play a very valuable role by helping clinical sites collect data. The FDA will use the data to determine if an investigational drug is safe and effective for use in our society.

Call (714) 831-1307 or Click here to ENROLL NOW
It takes less than 2 minutes to complete our form and someone from our staff will call you right away. There is absolutely no pressure to join and you can withdraw at any time. Our caring doctors will be happy to answer all of your questions.
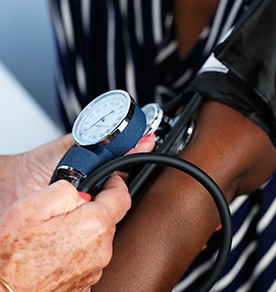
Complimentary Medical Consultation
Prior to joining one of our studies all individuals are provided with a complimentary medical consultation with one of our doctors. We will review your medical history and/or provide a free physical exam and blood test. There is no obligation to join a study!

No Special Requirements
You don’t have to be a U.S. citizen, you don’t need medical insurance and you don’t have to provide your social security number. You just need to be a human being that meets the inclusion criteria for our studies.

Compensation Provided
All participants are compensated for time and travel, which ranges from $100.00 to $2,500.00. Details of the compensation will be disclosed in the Informed Consent prior to participation. Snacks and meals are also provided for visits that take longer than two hours to complete.
What else do I need to KNOW?
All of our clinical studies are overseen by highly skilled and licensed doctors. Our studies are considered short term (1-14 months long) and are approved by the FDA and Institutional Review Board (IRB). All study applicants will be provided a consent form that outlines the risks and alternatives to joining a clinical study. Although study medication is provided, there is still the possibility of getting a placebo.


